Energy Industry Services: Mixed metallurgy pulp dryer - How to simultaneously control iron and copper corrosion?
05.10.2016
By Pekka Ojala, Product Group Manager, KL-Lämpö Oy
Boiler circulation control depends mainly on two factors: pH and oxygen (redox potential).
The control of the conditions gets even more critical while both iron and copper are present in the system. As pH is raised from pH > 8,9 the corrosion decreases for iron, but rapidly increases for copper (Fig. 1). This is especially true for ammonia. The protection of both the metals is however possible by proper selection of the alkalizing amines.
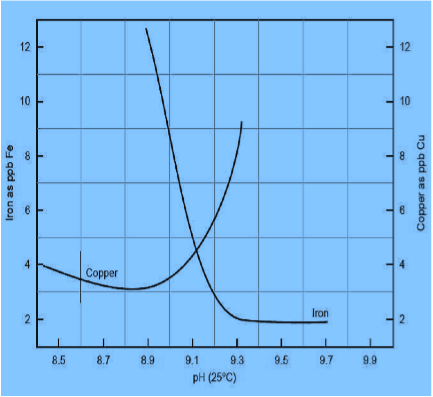
Even in recovery boilers, multifunctional chemical mixtures have been in use to control both oxygen corrosion and system pH. This system had been operated at rather low pH (8,9) in order to protect the copper alloy in pulp dryer batteries. This had in turn led to high iron levels, excessive oxide build up and need for acid cleaning.
Goals
The goals of the program development were:
1. Steam pH 9,2 - 9,3
2. Pulp dryer condensate copper < 5 µg/l
3. Feed water iron < 10 µg/l.
Conclusions
These goals may be reached by properly selecting the chemicals applied and dosing them for the need only. The primarytask is to maintain optimal system pH. In most cases, the dosage of oxygen scavenger needed is relatively low.
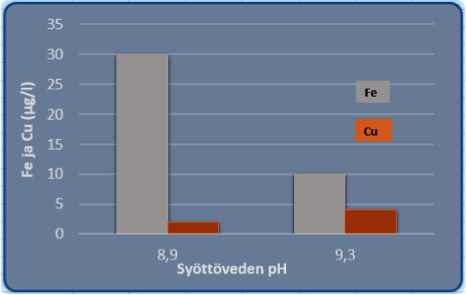
1. The feed water pH was raised
pH 8,9 9,35
2. The feed water iron was reduced
Fe 30 < 10 µg/l
3. The pulp dryer condensate copper remained at Cu < 10 µg/l
4. The boiler water iron level was reduced Fe 25 < 10 µg/l.

Benefits of the optimized chemical program
1. Higher system pH effectively inhibits iron corrosion
2. Copper concentrations remained under control. Normally, copper exchanger resin in the condensate system anyway cut the copper level to zero.
3. Iron oxide levels in the boiler circulation are dramatically reduced, thus minimizing scaling and deposits
4. The risk of under deposit corrosion and need of acid cleaning are reduced.
Read the case study
Download the report › (120 kb)



